The demand for high-performance rechargeable batteries has grown so considerably and universally in recent years that their various requirements and functionalities have almost become common knowledge. Among the various energy storage technologies, lithium batteries have emerged as an essential part of modern energy solutions, powering everything from everyday devices to electric vehicles. Their increasing popularity is due to their lightweight, high-energy density, and rechargeable nature. A conventional battery consists of an anode, a cathode, a separator, an electrolyte, and two current collectors, positive and negative. The anode and cathode serve as storage sites for lithium, while the electrolyte provides a medium for the intercalation of lithium ions across the separator between the anode and cathode during charge and discharge cycles.
In classical electrochemical systems, liquid electrolytes are frequently utilized due to their performance in aiding ion transport and improving electrode surface wetting. Most lithium-based electrolytes are made of highly flammable organic solvents, such as ethylene carbonate (EC), ethyl methyl carbonate (EMC), and diethyl carbonate (DEC). Under conditions such as overcharging, high temperatures, or leakage, these electrolytes can produce flammable gases, posing a considerable fire threat (Figure 2). The thermal degradation of batteries is tightly linked to their components, with the electrolyte playing a particularly essential role. Compared to electrodes or separators, the characteristics of the electrolyte are especially critical for assuring performance in high-temperature applications. Solid-state electrolytes (SSEs) offer a possible alternative, giving greater safety and improved electrochemical characteristics.
Solid-state electrolytes (SSEs) have a considerable safety benefit over liquid electrolytes. Energy density is commonly limited in conventional systems when high-voltage cathodes are applied due to the electrochemical instability of liquid electrolytes at high voltages. Furthermore, liquid electrolytes provide risks such as short circuits caused by dendritic growth on the anode surface during charging. SSEs are gradually being regarded as key components for next-generation batteries, overcoming the safety difficulties associated with liquid electrolytes. They have great mechanical strength, electrochemical stability, design flexibility, and excellent thermal stability. Furthermore, the higher energy density given by SSEs enables the fabrication of flexible and integrated battery designs, which is especially important for portable devices with optimum thickness. Solid-state batteries have the potential to impact hundreds of sectors by providing higher energy density, improved safety, and a longer lifespan than standard lithium-ion batteries. Furthermore, their functioning principle is the same as conventional lithium-ion batteries.
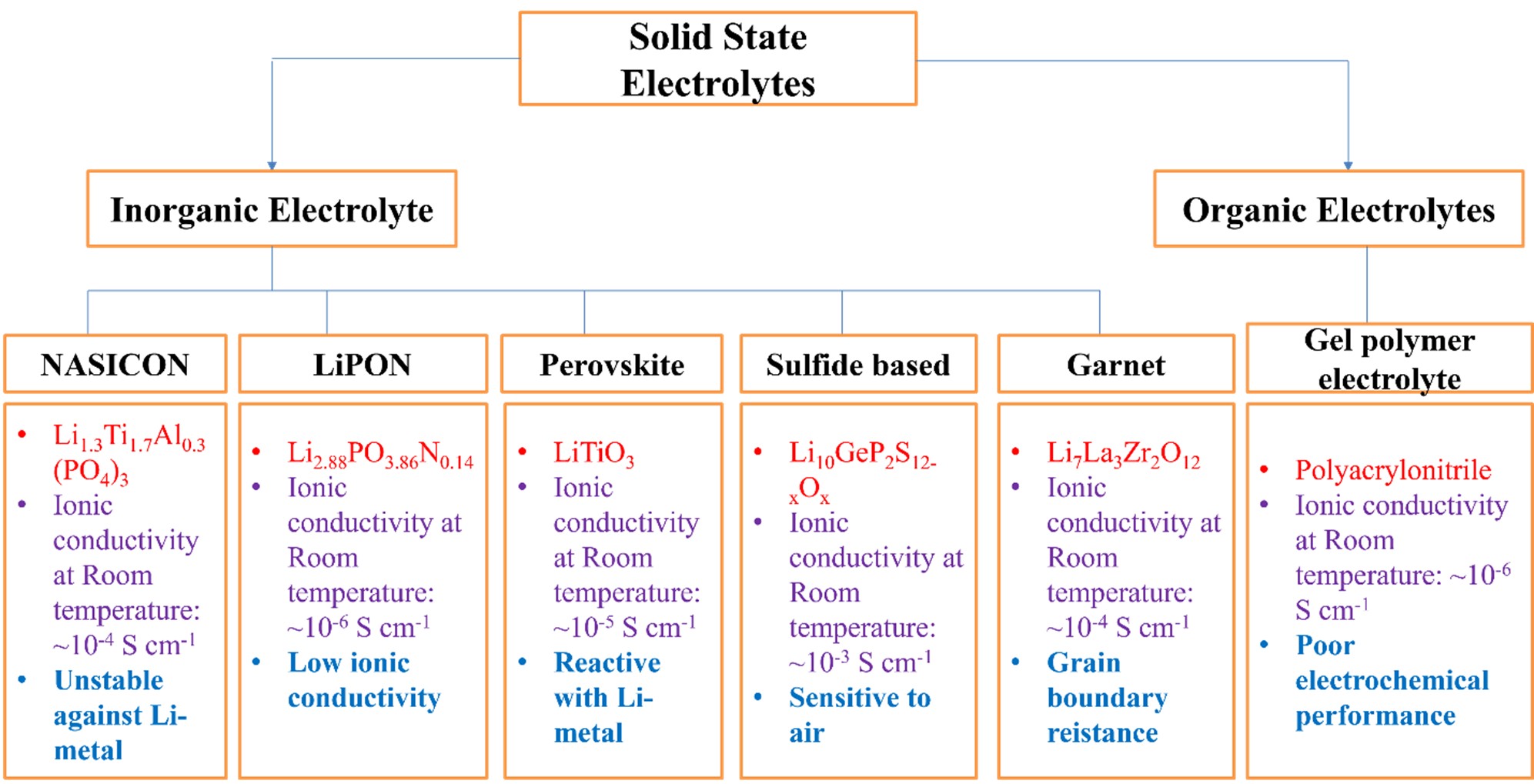
Solid-state electrolytes (SSEs) are broadly categorized into three types (Figure 1): (1) polymeric solid electrolytes (PSEs), (2) inorganic solid electrolytes (ISEs), and (3) composite-polymer electrolytes (CPEs). Among the ISEs, garnet-based lithium lanthanum zirconium oxide (LLZO) has garnered considerable attention due to its high ionic conductivity and excellent chemical stability when paired with lithium metal. The cubic and tetragonal phases are two different structural configurations observed in LLZO. Even though the phases have the same chemical composition, their characteristics differ significantly. Four more ordered and less mobile lithium sites per unit cell are present in the tetragonal phase, which is stable at lower temperatures. The decreased conductivity of lithium-ion (in the range of 10-6 to 10-7 S/cm) is caused by fewer effective conduction routes and fewer accessible lithium sites. On the other hand, the cubic phase, with eight lithium sites per unit cell, has a highly symmetrical structure that permits higher accessibility for lithium-ion motion. The cubic phase offers enhanced ionic conductivity, measured at 10⁻⁴ S/cm, and displays higher stability at high temperatures, making it more suited to complex energy storage applications.
Grain boundary resistance, however, continues to be a significant barrier to LLZO’s practical uses. Because of the crystalline structure’s intrinsic characteristics, it is essential to reduce the resistance that the microstructure’s grain boundaries introduce. Grain boundaries typically show a net decrease in the total ionic conductivity because they have lower ionic conductivity than the bulk. For LLZO to operate at its best and reach its full potential in solid-state battery applications, this problem must be resolved.
The work led by Associate Professor, Dr. Ghulam Ali, at the Advanced Energy Materials and Systems (AEMS) Lab, USPCAS-E, NUST, focuses on stabilizing the cubic phase of LLZO while managing the issue of grain boundary resistance. The research proposes an alternative method of employing lithium salt as a filler to lower grain boundary resistance in cubic LLZO. This approach boosts the lithium-ion transit across grain boundaries, hence enhancing the material’s overall ionic conductivity. The addition of lithium salt addresses the grain boundaries directly, providing more efficient ion-conducting channels. The new technique not only minimizes grain boundary resistance but also gives a different viewpoint for enhancing solid-state electrolytes. It offers enormous promise to increase the performance of solid-state batteries, opening the way for safer, more efficient, and high-performing energy storage devices.
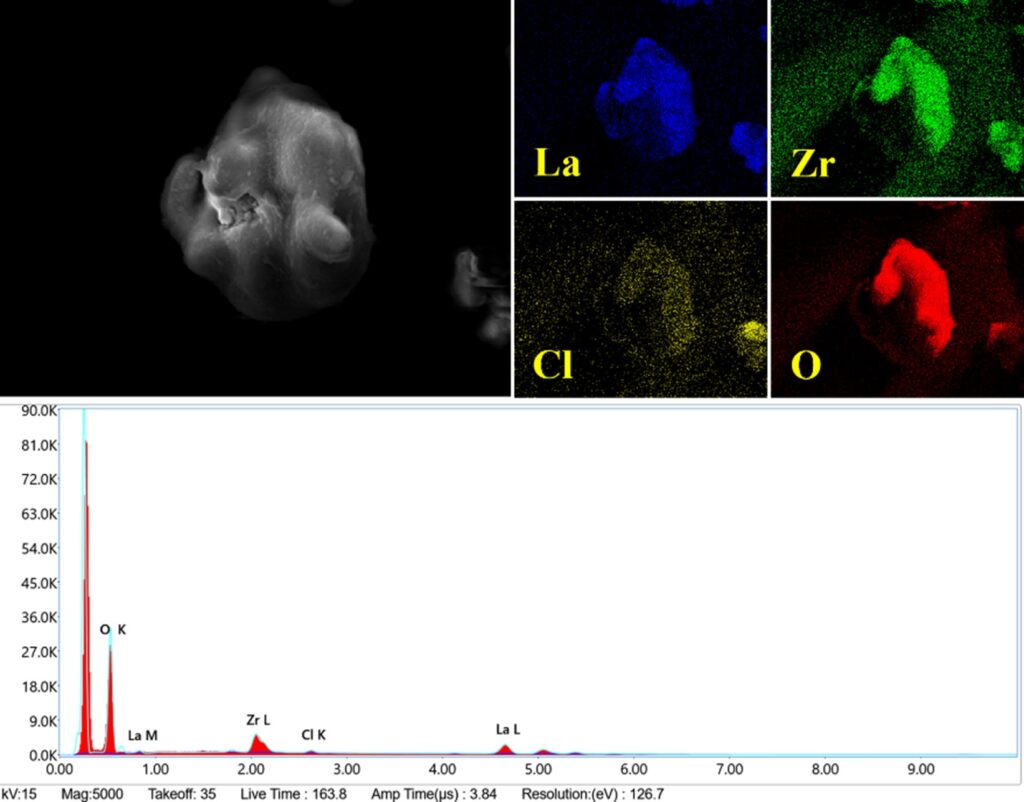
LLZO was synthesized utilizing a cost-effective solid-state reaction technique, which permits the large-scale manufacture of bulk LLZO. Electrochemical impedance spectroscopy indicated a grain boundary resistance of around 10,000 Ω and an ionic conductivity of 10−5 S/cm for the produced material. To boost performance, lithium salt was introduced as a filler into LLZO by a solution-based mixing procedure, guaranteeing uniform dispersion within the material and confirmed by energy dispersive X-ray spectroscopy (Figure 2).
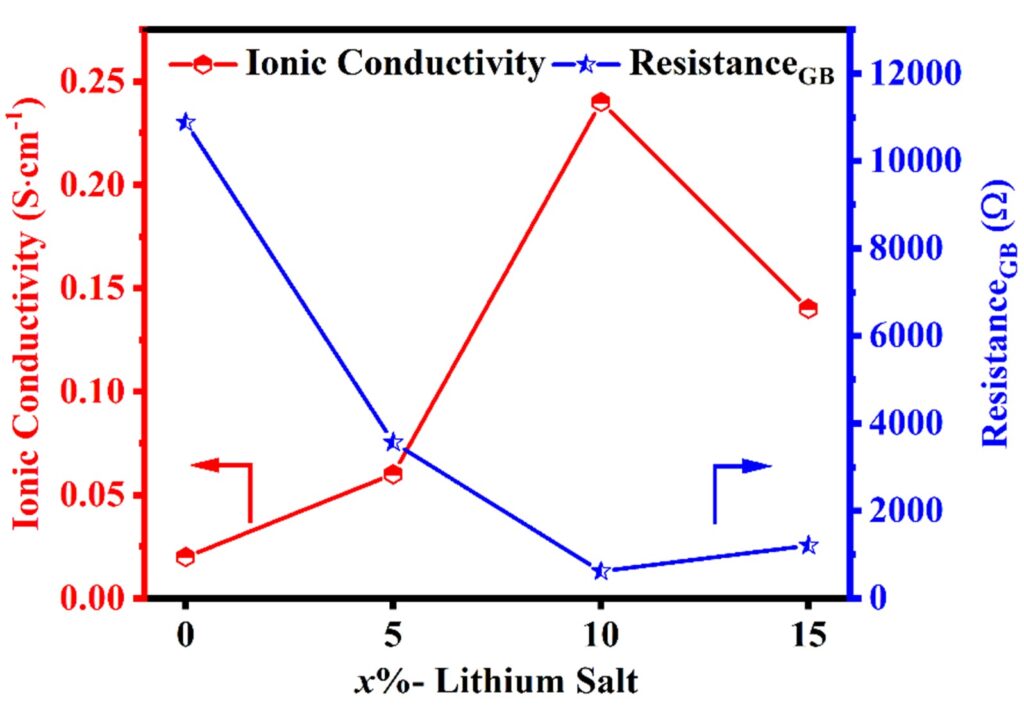
The optimized sample displayed a substantial improvement, obtaining an ionic conductivity of 10−4 S/cm, which is 10 times greater than that of pristine LLZO. Additionally, the grain boundary resistance was lowered by the same factor (Figure 3). Fabricated coin cells displayed the potential stability in a large voltage range of 0–5 V, coulombic efficiency of 87% after 50 charge-discharge cycles at a 1 C rate. The results emphasize the efficiency of the lithium salt filler in boosting the electrochemical performance of LLZO-based devices.
The outcomes of this research pave the path for prospective cooperation with the universities and industries devoted to promoting solid-state battery technology. Such partnerships may focus on scaling up the synthesis process and incorporating the improved LLZO electrolytes into commercial battery systems, therefore bridging the gap between research and actual applications.
The author is an Associate Professor at U.S.-Pakistan Center for Advanced Studies in Energy, National University of Sciences and Technology (NUST). He can be reached at ali@uspcase.nust.edu.pk.
Research Profile: https://bit.ly/42fzdXj

![]()