CO is a threat to human life and an environment pollutant [1]. It has become a challenge to aware of the dangers of CO poisoning. CO gas is the product of incomplete combustion of carbon containing material like natural gas, gasoline, kerosene oil, propane, coal, or wood. Artificial sources also produce CO which is colorless, odorless and tasteless [2]. Health effects of CO toxicity includes tissue hypoxia, syncope, seizures, hypotension, coma and chest pain. Extensive exposure to CO gas, symptoms may get worse that include vomiting, muscle weakness and collapse which may lead to death. CO toxicity also effects greenhouse gases such as CO2 , CH4 which in turn causes global warming and climate change [3].
Understanding the physiological and metabolic effects of CO toxicity is need of hours. Conventional CO gas sensors have been designed but requires high cost [4-5]. Prevalent metal oxide semiconductors with wide band gap considered for CO sensing hence low sensitivity [6-10]. Therefore, it is necessary to determine the best material and technology for detection and control of this toxic gas.
In materials research, the nanocluster science is an important area produced purposely through engineering for performing specific tasks and have applications in many industries such as health care, cosmetics, air purification. Nanostructured materials provide a large surface to volume ratio and tunable unique properties like high sensitivity, fast response time, and reusability so have gained much attention in technology over the bulk materials. Cluster encompass a range of geometries including semiconductors, among them (ZnO)n nanoclusters gained much attention due to its favorable morphology, structural and electrical properties. Semiconductor compounds like (ZnO)n are of particular importance due to its high excitation binding energy of 60 M eV, wide direct band gap energy (3.4-3.7) eV, and cost-effective in synthesis that’s enables its applications in photodetectors, transistors and environmental sensors. (ZnO)n sensors show high chemical and thermal stability, fast response time, good selectivity, shows remarkable sensing property when exposed to toxic gases due to its resistance change. Incorporating impurities in interstitials or vacancies of (ZnO)n nanocluster changes its morphology enhancing its sensing (electrical) properties.
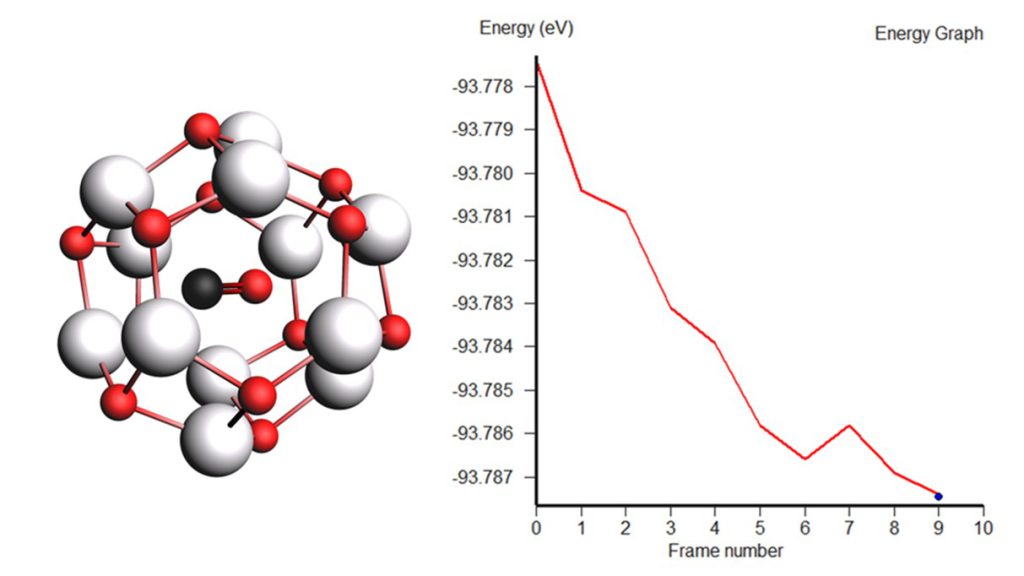
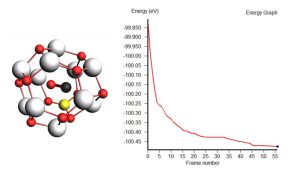
In our work the quantum chemical calculations of CO on Zn12O12 and Boron-doped Zn12O12 nanoclusters have been reported. The adsorption of CO on Zn12O12 and Boron-doped Zn12O12 nanoclusters have been studied and compared in terms of energy, geometry, electronic properties, HOMO-LUMO gap, charge transfer, DOS spectra, thermodynamic properties and simulated UV. The surface morphology and spectroscopic analysis were carried out for both cluster. Substituting Zn of Zn12O12 with Boron dopant was more favorable energetically. The electronic and thermodynamic properties favored Boron-doped Zn12O12 for CO adsorption due to reduction in energy gap after doped Boron. Boron-doped Zn12O12 as a CO gas sensor being focused due to its properties that are low cost, non-toxic, earth abundant, good doping effect, high surface to volume ratio and innovative synthesis which are the main concerns of research community nowadays. This flexible sensing material can be incorporated with smart sensing device for various applications such as automotive field, indoor air quality control, green house monitoring and for the comfort of wellbeing. This research project is a computational prototype that may open new doors to expanding nano applications in industry and technology and more importantly, a potential benefit to human quality of life.
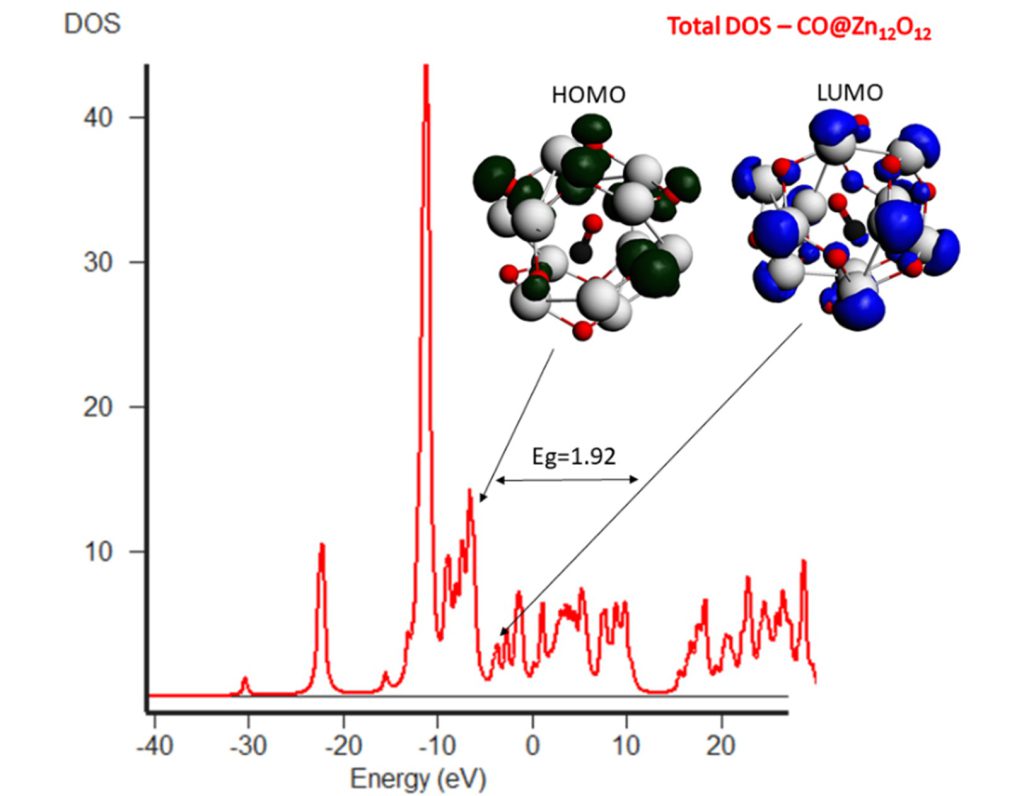
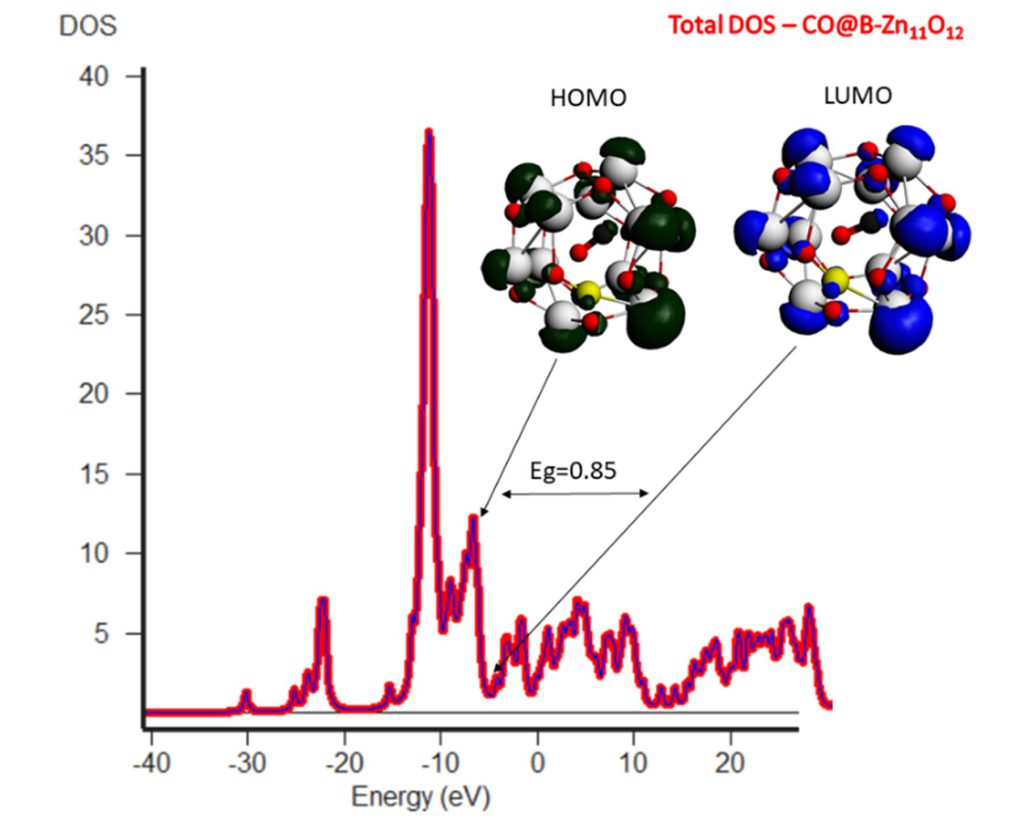
Introduction of dopant (boron) in Zn12O12 nanocluster, reduced the HOMO-LUMO gap hence the conductivity of nanocluster towards CO. The increasing entropy and negative value of ΔH and ΔG indicated that adsorption of CO over boron-doped Zn12O12 NC is energetically more favorable.
A significant change in energy gap is evidenced upon CO adsorption over boron-doped ZnO, indicating a higher charge transfer of 0.53 from boron-doped ZnO to CO. Hence it is cleared from the results that doping of nanocluster decreases energy gap and increases its reactivity towards CO gas thus enhancing sensing property of ZnO.
UV/Vis spectroscopic analysis reveal that the doping of boron results in the shifting of spectral range to fluorescent region depicting radiation emission by boron-doped Zn12O12 upon CO sensing and capturing. Concentration of CO gas molecules were increased and investigated the sensing behavior of boron-doped Zn12O12 nanocluster which showed that singly doped Zn12O12 NC can sense up to 5 CO molecules.
Mechanism of Sensing
Zn12O12 – metal oxide semiconductor taken as a gas sensor [36-37]. Boron doping shifted the spectral range from V is region to IR region, making it an IR absorbing material and CO is a typical gas which absorbs at IR. When CO is efficiently adsorbed, it shifted the spectral range which then generates a recordable signal. Through recorder, we can determine that boron-doped ZnO NC detected and captured CO gas.
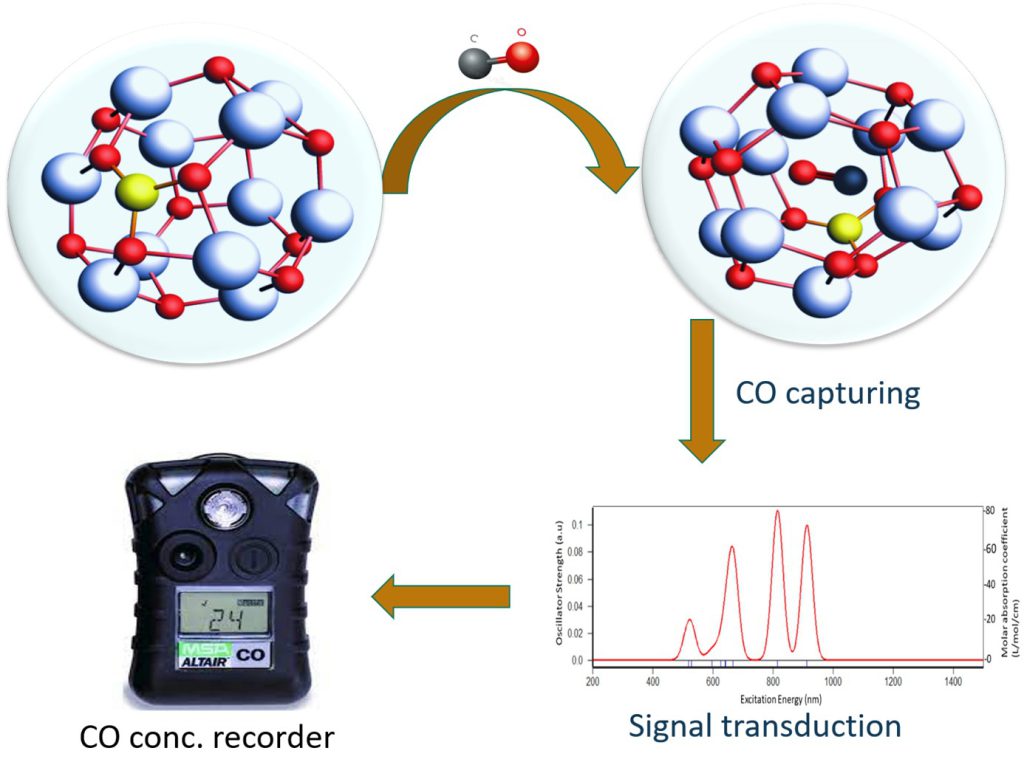
Current research is a step forward in understanding the nature of Zn12O12 nanocluster and it was concluded that boron doping reduced the energy gap and elevated stability upon adsorption of CO over Zn12O12 . Singly boron-doped Zn12O12 NC can detect and capture up to 5 molecules of CO gas efficiently.
The author is Associate Professor in the Department of Sciences at School of Interdisciplinary Engineering and Sciences (SINES), National University of Sciences and Technology (NUST). She can be reached at fouzia@sines.nust.edu.pk.
Researcher Profile: https://bit.ly/3WeJU74

![]()