Within the heart of NUST lies a state-of-the-art facility, devoted for the research, development and manufacturing of medical devices, where a dedicated team of biomedical scientists and engineers led by Dr. Murtaza Najabat Ali (Fig. 1) is striving for the betterment of healthcare facilities for the deprived population of Pakistan. Dr. Murtaza Najabat Ali, who is serving as Associate professor at SMME, Director MDDC and CEO NHT at NUST has been the brains behind the development of high-risk medical devices in Pakistan. With a vision to make Pakistan self-sufficient in the healthcare sector, the team is working tirelessly to devise solutions that are not only of superior quality but also affordable by even a lower-middle income household.
The facility consists of two divisions: Medical Devices Development Center (MDDC) Research Wing for the research and development of high-quality, cost-effective and indigenous medical devices, and N-ovative Health Technologies (NHT) – a Special Purpose Vehicle (SPV) established by MDDC-NUST – for the indigenous manufacturing and commercialization of medical devices in Pakistan. The production facility was inaugurated by the Prime Minister, Imran Khan, along with other federal ministers on October 16th, 2021. The NHT facility has been established as a public sector company registered with the Securities Exchange & Commission of Pakistan (SECP), is ISO 13485 certified from European Commission notified body and is licensed by Drug Regulatory Authority of Pakistan (DRAP).

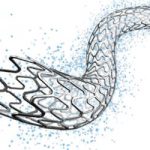
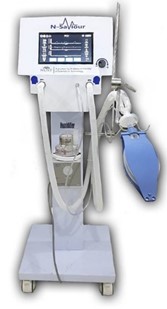
Since inception (2017), a number of medical devices and production/testing equipment have been developed at MDDC by the team, trained and certified from the European partners. The medical devices that are currently being manufactured at NHT include: bare-metal stents (BMS, Rejuvenate®) and Percutaneous Transluminal Coronary Angioplasty (PTCA) catheters (Vasoglide®). More than 500 stents and balloon catheters have been supplied in different hospitals of Pakistan. Drug eluting coronary stents (DES), Diagnostic Angiographic catheters, Ventilators and Powered Air Purifying Respirators (PAPR) are in the final stages of product development. The medical devices that are currently being manufactured or developed by MDDC-NHT are shown in Figures 2-8.




1. Grants Awarded:
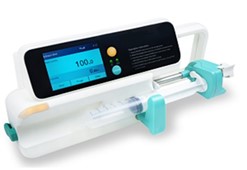
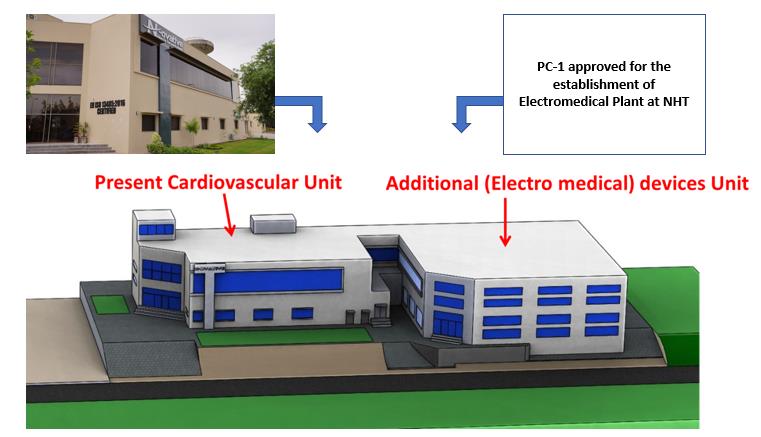
During the wake of COVID-19, a dedicated team of engineers at MDDC-NHT worked diligently to develop cost-effective and indigenous ventilator, PAPR and many other electromedical devices. Recently, PC-1 amounting Rs. 396.396 Million for the establishment of the electromedical plant was approved by MoST. In this plant, the electromedical devices like ventilators, PAPR, Syringe pumps and Oxygen concentrators will be manufactured for commercialization.
Pakistan is completely dependent on imported electromedical devices. Government of Pakistan is spending about $3.04 billion on healthcare annually, and hence the indigenous development and production of these electromedical devices along with the production and testing equipment will offer a massive relief to the Government of Pakistan by cutting down the foreign exchange (FE). Along with this, the high quality, inexpensive medical devices will be accessible to even a lower middle-income household, thus improving the life quality and expectancy of the patients.
2. Technology & Research Innovation Activities at MDDC-NHT
The Process Technology Wing works directly under the MDDC research wing. It houses highly skilled team of engineers engaged in the indigenous development of the production and testing machines in order to develop capacity building within the country. With this wing, the aim is to enhance the production capacity of medical devices as well as to achieve self-reliance in future. Some of the process technology equipment that have been developed by the team include;
a. Stent Fatigue Testing Machine
This machine has been designed to evaluate the fatigue experienced by the stent over a certain period of time, under accelerated simulated conditions.

b. Catheter Trackability Testing Machine (CTTM)
This machine has been designed to evaluate the trackability (the force required to move a catheter inside the blood vessel to the target site) by simulating the complex anatomical human blood vessel model. This machine is equipped with a dedicated and completely indigenous user-interface.

c. Diagnostic Catheter Testing Bench
This testing bench has been designed to evaluate the performance of diagnostic angiography catheters. It is equipped with temperature-controlled bath and dry zone for testing the burst pressure rating and any leakage present.

d. Stent Visual Inspection Machine
The machine has been designed to aid the visual inspection of stents which is a requirement for quality control.

e. Stent Drug Coating Machine
The ultrasonic spray coating machine for depositing the drug on the surface of stents is another machine that has been developed indigenously at the MDDC-NHT facility.

f. Flexural Strength Testing Machine
Flexural test bench is designed to test the flexibility of the catheter, it caters both, Diagnostic Angiographic Catheter (DAC) and Percutaneous Transluminal Angioplasty Catheter (PTCA).

g. Contact-based Catheter Coating Machine
This machine utilizes the principle of wipe coating to apply the hydrophilic solutions on the surface of catheters. The pads, loaded with the hydrophilic solution, traverse over the length of the catheter in order to render the surface of the catheter more hydrophilic. Catheters with hydrophilic surfaces experience less resistance in the blood vessels and are less likely to cause injury or perforations, making the diagnostic or interventional procedure less painful.

h. Non-Contact Based Catheter Coating Machine
This machine utilizes the principle of ultrasonic spray coating to coat the surface of the catheter with hydrophilic solution.

Pakistan is completely dependent on imported electromedical devices. Government of Pakistan is spending about $3.04 billion on healthcare annually, and hence the indigenous development and production of these electromedical devices along with the production and testing equipment will offer a massive relief to the Government of Pakistan by cutting down the foreign exchange (FE). Along with this, the high quality, inexpensive medical devices will be accessible to even a lower middle-income household, thus improving the life quality and expectancy of the patients.
3. Research Paper Published
MDDC-NHT recently published a research paper titled, ‘Preclinical Evaluation of the Vascular Effects of Rejuvenate® Cobalt Chromium Coronary Stent System Implanted in the Porcine Coronary in Stent Restenosis Model’ [1] in Pakistan Heart Journal on June 24th, 2021. The research paper disseminates the results of the animal trials conducted at the Center for Cardiovascular Research and Development, American Heart of Poland. This trial was performed to test the safety and effectiveness of NHT’s Bare metal stent (Rejuvenate®) in the porcine model of coronary in-stent restenosis.
4. Talks delivered by Dr. Murtaza Najabat Ali – Director MDDC and CEO NHT
a. Medical Devices and In vitro Diagnostics
As a keynote speaker, Dr. Murtaza Najabat Ali shed light on his journey – from idea to conception and reality, and enlightened the audience with his venture. This life sciences webinar was held on March 13th, 2021.
b. Reflection of COVID-19 and Way Forward
Dr. Murtaza Najabat Ali was invited as a speaker in a two-day international seminar, titled: ‘Reflection of COVID-19 and Way Forward’, held at Government College University Hyderabad on April 9th and 10th, 2021. The theme of the seminar was ‘Technology, Innovation and Socio-economic Activities’. Dr. Murtaza Najabat Ali briefed the audience about the products developed by MDDC-NHT and the projects MDDC-NHT is working on to overcome the COVID-19 crisis.
c. Design Innovation and Healthcare Technology in Pakistan, NUST
This webinar was arranged by APPNA Medical Education, Research, International Training and Transfer of Technology (APPNA MERIT) in collaboration with Research, Education & Scientific Affairs (RESA) Committee on June 12th 2021. Dr. Murtaza Najabat Ali shared his journey with the audience and briefed them about how the team at MDDC-NHT took the matter into their own hand after seeing Pakistan’s situation in the Covid-19 crisis and how they left no stone unturned to develop N-Savior (mechanical ventilator) and NUST-PAPR (Powered Air Purifying Respirator) in the limited resources during the 2020 lockdown. He also stated that the MDDC-NHT facility is actively working on some projects and will have up to 10 products in the market by the end of 2024.
References
[1] Ali, M. N., Mir, M., & Buszman, P. (2021). Preclinical Evaluation of the Vascular Effects of Rejuvenate® Cobalt Chromium Coronary Stent System Implanted in the Porcine Coronary in Stent Restenosis Model. Pakistan Heart Journal, 54(2), 132-138. https://doi.org/10.47144/phj.v54i2.2087
This author is serving as Assistant Manager Research at MDDC NUST. She has a vast experience in biomedical research with a focus on Cardiovascular Devices, Electromedical Devices, Diabetes Management, Drug delivery systems and Biomaterials. She can be reached at amr@mddc.nust.edu.pk.





