In countries like Pakistan where research commercialization is a new concept, NUST is already recognized for its efforts to develop marketable innovations to benefit the society. It is home to many eminent scientists and leading researchers like Professor Dr Murtaza Najabat Ali who are working tirelessly to conduct applied research and strengthen academia-industry linkages. Being a distinguished Biomedical Engineer, Dr Murtaza is known for taking the initiative of establishing Pakistan’s first research-driven medical devices manufacturing industry at NUST for producing cardiovascular and electromedical devices indigenously, thus setting precedence for other universities and researchers. He is presently leading Medical Devices Development Center (MDDC), N-ovative Health Technologies (NHT), Prosthetics and Implantology Lab SMME at NUST. The triad, under his leadership, is continuously conducting research and innovation for good health and well-being (SDG 3) of Pakistani Population. In this quarter, his team (Project Lead: Dr Murtaza Najabat Ali, Team members: Ramsha Munir and Zunaira Qureshi) from the prosthetics and implantology lab group developed a novel pain-reliving topical formulation for the management of sprains, strains, tendonitis and arthritis pain.
Musculoskeletal pain represents a substantial health problem, with an estimated 20–33% of people globally living with painful musculoskeletal conditions. The cost on managing pain is exorbitant not only for the individual but for the economies as well. Moreover, the best commercially available topical analgesic (Voltral Emulgel) has Number Needed to Treat (NNT) 1.8. This means if 10 people apply this treatment, only 5 of them will have their pain relieved, while the other 5 will continue to suffer. That been said, the information on NNT of other topical analgesics has not been reported in the literature and these analgesics are believed to have NNT much less than that of Voltral Emulgel. Therefore, there is not only a need for devising novel analgesic active pharmaceutical ingredients but also enhancing the drug release rate of already available formulations.
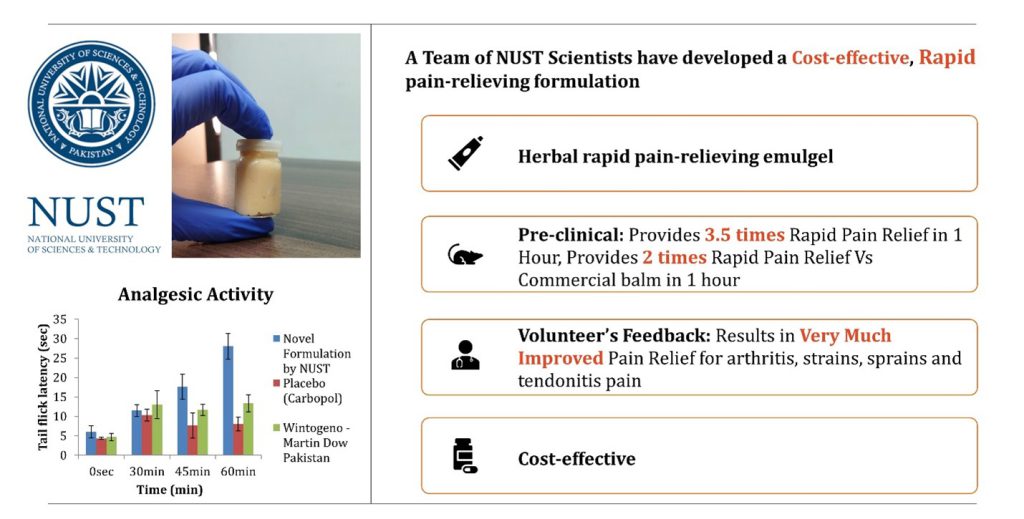
The team from SMME NUST developed the novel formulation to address this problem area. This milestone, where a commercially available balm was transformed into a rapid analgesic formulation, was achieved by the addition of some Active Pharmaceutical Ingredients (API) and excipients and by tailoring the stoichiometric ratios of the composition. The ingredients and approaches used in this novel medicament builds on years of safety and clinical experience. The formulation was then subjected to extensive in-vitro and preclinical test and trials to evaluate its physicochemical properties, stability, accelerated shelf-life, efficacy and safety.

The formulation exhibited promising results in the in-vitro and preclinical test and trials. It provides 3.5x more rapid pain relief vs placebo and 2x more rapid pain relief vs commercial Wintogeno balm in 1 hour when tested on animal model. Additionally, no sign of dermal irritation was evidenced within 24 hours of application. Some volunteers also tried the emulgel to manage their pain. According to the Patient Global Impression of Change (PGIC) score, the formulation resulted in a very much improved pain relief for most of the volunteers suffering from acute musculoskeletal pain (strains, sprains, tendonitis) and arthritis pain. The volunteers’ comments received are shown in Figure 3:

The team believes that this novel formulation may be marketed as a cost-effective solution for pain-management as the per-unit raw-material cost on this formulation is small and a further decrease in cost is expected because of economy of scale.
Remarks by Project Lead
Based on the promising characterization and in-vitro testing results, comparative study with predicate formulations and the feedback from volunteers, the novel formulation is expected to suffice the unmet need of an effective and rapid pain reliever while being cost-effective. Therefore, NUST Team is ready to license this novel formulation to some pharmaceutical industry so that the formulation may reach the beneficiaries without delay.

The author is working as Assistant Manager Research at Medical Devices Development Center (MDDC) established at National University of Sciences and Technology (NUST). She has a vast experience in biomedical research with a focus on Cardiovascular Devices, Electromedical Devices, Diabetes Management, Drug delivery systems and Biomaterials. She can be reached at amr@mddc.nust.edu.pk.

![]()





