Epilepsy is a common neurological disease with a toll of 50 million people diagnosed annually worldwide. The rate of Epilepsy is likely to be higher in low-income countries due to lack of proper treatment mechanism (WHO), which results in 90% increase of disease in some countries. According to an estimate, 70% of people with epilepsy could be seizure free if properly diagnosed and treated (WHO). NUST, once again takes pride in developing an innovative technology named as EPIC (Epilepsy Care) for smart monitoring of epilepsy. The intellectual property of this technology was protected and licensed out (04x IPRs) to RiseTech (Pvt) Ltd, making the total count of IPRs transferred from NUST to industry at 40.
EPIC (Epilepsy Care) is a smart Epilepsy monitoring and notification device which is designed to connect Epilepsy patients with their caregivers whenever patients experience Generalized Tonic-Clonic (GTC) seizures. Approximately 50 million people in world have epilepsy according to the report of world health organization and Pakistan has 5% of world epilepsy population, which makes it approximately 2.5 million people. Considering the fact that seizures are unprovoked and can occur at any time, many patients find it difficult to carry out their normal daily life activities due to state of uncertainty that prevails among them. Physical injuries are common in such patients. These injuries include head, dental injury, burn, fractures, road accidents and submersions. Most of them can be prevented if properly notified and predicted. The patient can then get the first aid in case of seizure onset or can avoid physical activity resulting in such injuries.
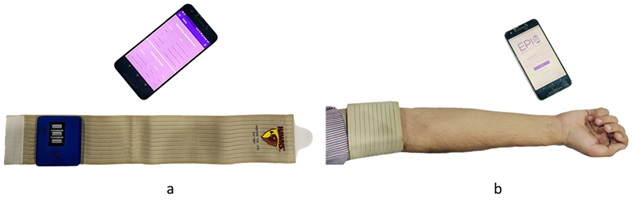
Our product consists of a non-invasive wearable band for continuous monitoring of seizure activity along with assistive mobile and web applications for the caregiver and doctor. The device reads muscle activities and with the help of advance signal processing and machine learning algorithms, it identifies any GTC activity from normal activities. The band does not focus on prediction of seizure rather focuses on logging of information and notification to caregivers. Thus, it is used for the purpose of monitoring and logging the abnormal/seizure activity of the patient during daily routine work. Application logs frequency of seizure, duration and time of seizure on a cloud platform, which can help the doctor in accurate assessment. The application is available on Google Play store. In this research work, 04 intellectual property rights (patent, industrial design and copyrights) have been filed. Our device has also won Pakistan Software House Association (P@SHA) award. EPIC’s proof of concept and prototype is ready and we are currently in process of finalizing Institutional Review Board (IRB) with Shifa International Hospital for detailed clinical trials of device.
The team includes innovators Dr. Sajid Gul Khawaja (Assistant Professor), Dr. Muhammad Usman Akram (Associate Professor), and Mr. Ali Saeed Khan (Lecturer) from College of Electrical and Mechanical Engineering, National University of Sciences and technology (NUST), Islamabad, Pakistan.
The author is the Assistant Professor at College of Electrical and Mechanical Engineering, National University of Sciences and Technology (NUST). He can be reached at sajid.gul@ceme.nust.edu.pk

NUST Research, Defining Futures, Research Activities in Pakistan, Epilepsy Treatment NUST, Epilepsy Seizure NUST, Epilepsy in Pakistan NUST, Epilepsy Neurological Disorder NUST




