The research and innovation activities by Dr Murtaza Najabat Ali and his team were in the limelight yet again during this quarter, as the team worked tirelessly to achieve the aim of taking NUST and Pakistan to the new heights of success. Briefly, the team secured a research grant worth 7.4 million, published three papers and filed one patent. The construction of Pakistan’s first electromedical devices production unit has begun under the flagship of Medical Devices Development Center (MDDC) which is Pakistan’s first research based medical devices development facility. In this production unit, the electromedical devices like Ventilator, Powered-air purifying respirator (PAPR), syringe pumps and oxygen concentrators will be manufactured. Dr Murtaza Najabat Ali is working as an Associate Professor at School of Mechanical and Manufacturing Engineering (SMME), Project Director at Medical Devices Development Center (MDDC) and Chief Executive Officer at N-ovative Health Technologies in the National University of Sciences and Technology.
1.1 Construction of Pakistan’s first electromedical devices production facility began at MDDC / NHT
An additional production facility for manufacturing electromedical devices; Ventilators, Powered Air Purifying Respirators (PAPR) and the like, is being established at MDDC. The establishment of this facility is funded through a PC-1 amounting Rs. 396.396 million approved by Ministry of Sciences and Technology (MoST). In this plant, the electromedical devices like ventilators, PAPR, Syringe pumps and Oxygen concentrators will be manufactured for commercialization.
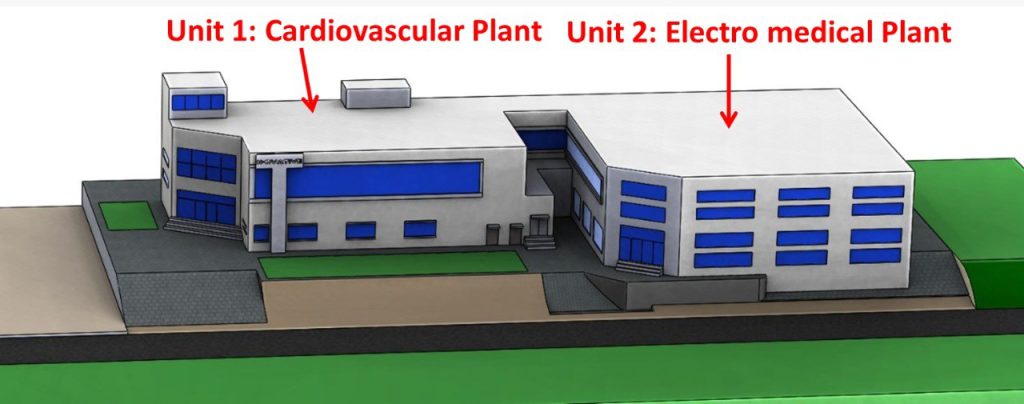

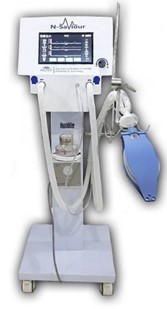

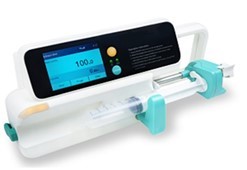
Pakistan is entirely reliant on imported electromedical supplies. The government of Pakistan spends roughly $3.04 billion on healthcare each year, therefore the indigenous research and production of these electromedical devices, as well as the manufacturing and testing equipment for the same, will provide significant relief to the government by reducing foreign exchange (FE) expenditure. Additionally, high-quality, low-cost medical devices will be available to the patients belonging to even lower-middle-income families, thereby boosting the patients’ quality of life and life expectancy.
1.2 Research Grants Won
Rs. 7.4 million have been granted by Pakistan Science Foundation (PSF) for the design and development of Powered Air purifying Respirator (PAPR) prototype. This grant would be beneficial in expediting the development of PAPR for production in the electromedical unit of MDDC/NHT.
1.3 Dissemination Activities
During this quarter, one patent was filed and three papers were published.
- ‘An insight into Potential Pharmacotherapeutic Agents for Painful Diabetic Neuropathy’ in Journal of Diabetes Research (Impact factor: 4.011). The authors are Zunaira Qureshi, Murtaza Najabat Ali and Minahil Khalid.
Painful Diabetic Neuropathy (PDN) is a devastating complication of Diabetes and about one-third of the diabetic population experience this complication in their lifetime. In this paper, the authors compiled the research activities that have been done vis a vis Painful Diabetic Neuropathy (PDN) till date. PDN results in an unimaginative agonizing pain whenever clothes touch the patients’ skin (allodynia), unprompted burning sensation like boiling water, pricking of “pins and needles” in feet when the patient walks along with other painful symptoms. This along with sleep disturbance and the constant pressure of managing normal blood glucose results in anxiety and depression in patients. The available therapeutic options are not good enough as they only provide partial pain relief, that too, in one-third of the patients. The reason for unavailability of effective treatment options for PDN is our partial understanding of the pathways that cause it. This paper highlighted this problem and summarized the scientific advancements done till date to understand the underlying cause of this disorder. The authors also briefed about the potential pharmacotherapeutic agents for PDN [1].
![Figure 6: Clinical appearance of the most prevalent type of diabetic neuropathy and progression of sensory loss over the course of PDN. Adapted from [2] (CC BY 4.0). During the course of the disease, the loss of sensory functions, in the regions colored as blue and red, occurs and the patient feels unbearable pain in these regions](https://researchblog.nust.edu.pk/wp-content/uploads/2022/04/Figure-6-1024x941.jpg)
- ‘Synthesis and in vitro Evaluation of Natural Drug loaded polymeric films for cardiovascular applications’ in Journal of Bioactive and Compatible polymers (Impact factor: 1.756). The authors are Bakhtawar Ghafoor and Murtaza Najabat Ali.
Heart Diseases are the leading cause of deaths worldwide. To avoid dreadful consequences and mortality, a percutaneous coronary intervention (PCI) is performed during which a stent is implanted in the patients. In this research, a biodegradable polymeric composition comprising of natural drug was developed for coating on a third-generation Drug Eluting Stent (DES). The results of the lab-based studies revealed the significant anticoagulation and antioxidant properties of the developed composition to address cardiovascular disorders such as atherosclerosis, restenosis, and thrombus formation. It was concluded that the drug-loaded polymeric compositions have the potential to be used as a coating material for biomedical implants after extensive testing on animal model and clinical trials [3].
- ‘Evolutionary Perspective of drug eluting stents from thick polymer to polymer free approach’ in Journal of Cardiothoracic Surgery (Impact factor: 1.470). The authors are Sadia Hasan, Murtaza Najabat Ali, Bakhtawar Ghafoor.
This review paper highlights the major transitions in stent technology evolution from Bare-metal Stents (BMS) to Drug Eluting Stents (DES), from thick polymeric coatings to thin coatings, from durable polymers to polymer free drug eluting stents. It began when the stainless-steel stents with thicker drug-polymer coatings were used in the first-generation DES. Despite their better results than the BMS, the quest for introducing an even better option continued. The second-generation stents followed, which comprised of superior metal platforms, thinner struts, and thin coatings. The drugs in these DES were also changed, i.e., Paclitaxel and Sirolimus were replaced by Zotarolimus and Everolimus. These stents outperformed the predicate devices, but there was a problem with the permanent coating, which remained intact across the stent surface after complete drug release and exhibited problems in the longer run. To avoid these problems, a better version of DES was introduced, known as the third generation of drug eluting stents, which used biodegradable polymer at first and eventually switched to polymer-free drug coatings. To accomplish the polymer-free coatings, researchers have drawn on to a unique combination of technologies and extensive research is being carried out in this domain [4].
![Figure 7: Polymer-free third generation DES A Attributes of 3rd generation DES B Design of commercially available polymer free DES C Strategies of drug coating in polymer free DES. Adapted from [4] (CC BY 4.0)](https://researchblog.nust.edu.pk/wp-content/uploads/2022/04/Figure-7.jpg)
Remarks
Team MDDC/NHT under the leadership of Dr Murtaza is leaving no stone unturned to bring prestige to NUST and Pakistan. The team envisages a future in which Pakistan is capable enough to discontinue importing medical devices and the people of Pakistan have access to high-quality and up-to-date healthcare facilities at an affordable price. This will strengthen the economy on one hand and on the other hand will improve the life quality of citizens of Pakistan.
References
[1] Qureshi Z, Ali MN, Khalid M. An Insight into Potential Pharmacotherapeutic Agents for Painful Diabetic Neuropathy. Journal of Diabetes Research. 2022 Jan 27;2022. DOI: 10.1155/2022/9989272
[2] S. S. Gylfadottir, D. Weeracharoenkul, S. T. Andersen, S. Niruthisard, S. Suwanwalaikorn, and T. S. Jensen, “Painful and non-painful diabetic polyneuropathy: clinical characteristics and diagnostic issues,” Journal of Diabetes Investigation, vol. 10, pp. 1148–1157, 2019.
[3] Ghafoor B, Najabat Ali M. Synthesis and in vitro evaluation of natural drug loaded polymeric films for cardiovascular applications. Journal of Bioactive and Compatible Polymers. 2022 Mar 12:08839115221085735. DOI: 10.1177/08839115221085735
[4] Hassan S, Ali MN, Ghafoor B. Evolutionary perspective of drug eluting stents: from thick polymer to polymer free approach. Journal of Cardiothoracic Surgery. 2022 Dec;17(1):1-20. DOI: 10.1186/s13019-022-01812-y
This author is serving as Assistant Manager Research at MDDC NUST. She has a vast experience in biomedical research with a focus on Cardiovascular Devices, Electromedical Devices, Diabetes Management, Drug delivery systems and Biomaterials. She can be reached at amr@mddc.nust.edu.pk.






